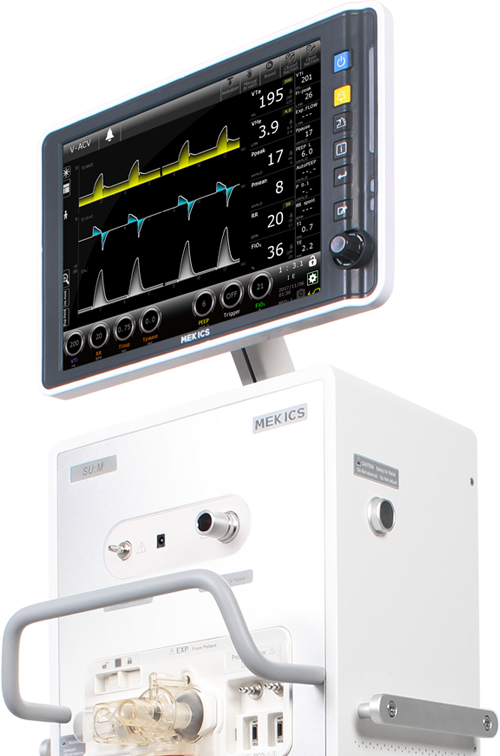
OVERVIEW
MEKICS has taken the lead of developing and commercializing
ventilators based on Korea’s first and unique respiratory technologies.
ventilators based on Korea’s first and unique respiratory technologies.
We are proactive in expanding the application of our respiratory care solutions developed by various research activities in order to help more patients.
This effort will allow us to expand our top-notch technologies to both hospital and homecare sectors with the presentation of various evidences on how the demographic and environmental changes have effects on respiration and health of the mankind.
MEKICS has a strong belief that product safety and quality of raw materials must in no way be compromised.
To this end, we have established internal systems and R&D bases satisfying the quality and safety standards for the functionality of respiratory care products. Those help us correctively understand overseas local market circumstances and build a technologically cooperative agent system, thereby contributing to MEKICS evolving into a global player in the respiration care market.
This effort will allow us to expand our top-notch technologies to both hospital and homecare sectors with the presentation of various evidences on how the demographic and environmental changes have effects on respiration and health of the mankind.
MEKICS has a strong belief that product safety and quality of raw materials must in no way be compromised.
To this end, we have established internal systems and R&D bases satisfying the quality and safety standards for the functionality of respiratory care products. Those help us correctively understand overseas local market circumstances and build a technologically cooperative agent system, thereby contributing to MEKICS evolving into a global player in the respiration care market.
ACHIEVEMENTS
Certification of CE, ISO etc. through continuous R&D of MEK