SEOUL, South Korea — Renowned medical device enterprise, MEKICS Co., Ltd. (CEO: Kim Jong-Cheol), a leader in the field of artificial respirators and respiratory therapeutic devices, proudly announced the acquisition of the European Medical Device Regulation (CE MDR) certification for its next-generation respiratory therapeutic device OmniOx 'HFT750' on August 24, European time.
The CE MDR, effective from May 2021, replaces the previous European Medical Device Directive (CE MDD). It introduces strengthened criteria related to safety, performance, and efficiency. Particularly notable is that MEKICS has achieved the Class IIb level in this certification — an accomplishment considered challenging in the respiratory therapeutic device sector. This marks the company as the first in South Korea to attain this recognition within the domain.
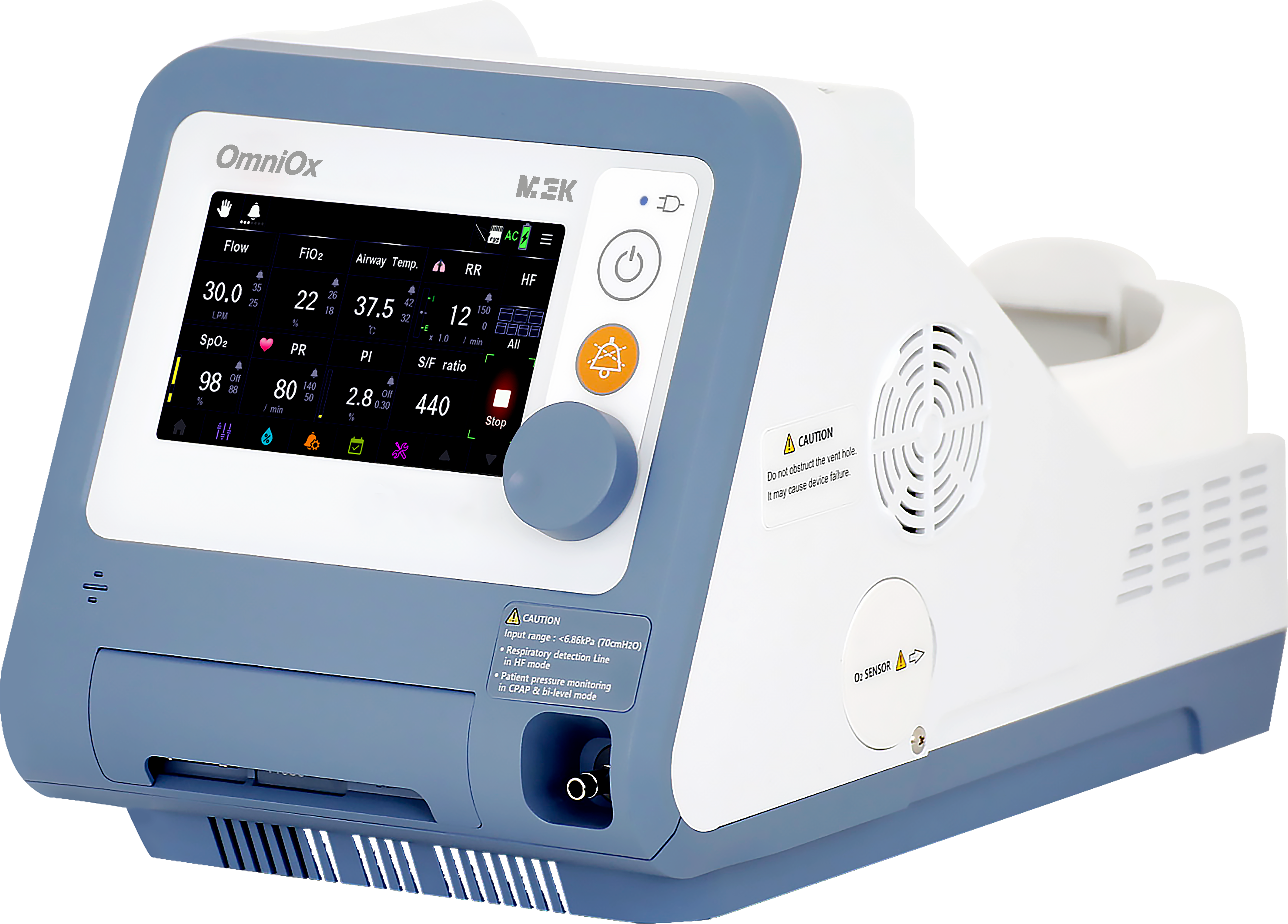
OmniOx 'HFT750' stands out as an innovative medical device that offers both non-invasive ventilation and high-flow nasal cannula therapy within a single device. Remarkably, it ensures continuous patient care even during patient transportation, thereby enabling efficient oxygen therapy for a majority of patients, excluding those critically ill requiring intubation.
Kim Jong-Cheol, CEO of MEKICS, expressed his confidence in the OmniOx HFT750, describing it as a "groundbreaking product offering continuity in respiratory care and convenience in airway management, particularly relevant during the COVID-19 pandemic." He further stated, "The MDR certification for HFT750 sets a benchmark for entering the global market with a product that has the potential to transform the paradigm of respiratory therapy. We anticipate this as an opportunity to preemptively capture the evolving market."
Having proven its advanced technical prowess and product quality through this certification, MEKICS is poised to expand its market presence. With plans to participate in renowned conventions and exhibitions like the World Congress of Intensive Care (WICC) and the European Respiratory Society (ERS), the company aims to fortify its sales foundation in the European region. Furthermore, as a specialized respiratory therapeutic device company with cutting-edge technology, MEKICS endeavors to elevate its global brand recognition.
***




